Site-selective phenol acylation mediated by thioacids via visible light photoredox catalysis†
Abstract
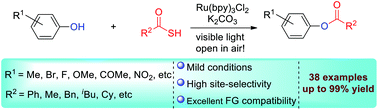
Site-selective phenol acylation mediated by thioacids with various phenols as acylation surrogates via visible light photoredox catalysis is described. This protocol provided facile access to an array of phenolic esters, with the exclusive acylation of a phenol hydroxyl group over an alcoholic one. The utility of this methodology was also demonstrated by the modification of biologically meaningful natural products.


 Please wait while we load your content...
Please wait while we load your content...