Tandem Prins-type cyclization for the stereoselective construction of fused polycyclic ring systems†
Abstract
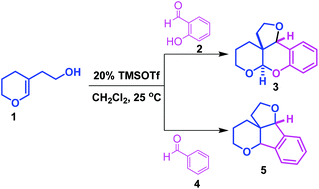
A novel strategy has been developed for the synthesis of fused tetrahydrofuro[3,2-c]pyrano[2,3-b]chromene derivatives through the condensation of 2-hydroxybenzaldehydes with 2-(3,4-dihydro-2H-pyran-5-yl)ethan-1-ol in the presence of 20 mol% TMSOTf in dichloromethane at 25 °C. Similarly, the coupling of aromatic aldehydes with 2-(3,4-dihydro-2H-pyran-5-yl)ethan-1-ol provides the corresponding hexahydro-4H-furo[3′,2′:2,3]indeno[1,2-b]pyran derivatives in good yields with high diastereoselectivity. It is a modular approach for the rapid construction of polycyclic architectures in a single step. These cyclic frameworks are an integral part of the structure of many natural products.


 Please wait while we load your content...
Please wait while we load your content...