Manipulating the positions of CH⋯N in acceptors of pyrimidine–pyridine hybrids for highly efficient sky-blue thermally activated delayed fluorescent OLEDs†
Abstract
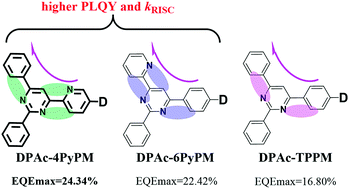
Three novel TADF materials, 10-(6-(2,6-diphenylpyrimidin-4-yl)pyridin-3-yl)-9,9-diphenyl-9,10-dihydroacridine (DPAc-4PyPM), 9,9-diphenyl-10-(4-(2-phenyl-6-(pyridin-2-yl)pyrimidin-4-yl)phenyl)-9,10-dihydroacridine (DPAc-6PyPM) and 10-(4-(2,6-diphenylpyrimidin-4-yl)phenyl)-9,9-diphenyl-9,10-dihydroacridine (DPAc-TPPM) have been designed and synthesized. CH⋯N intramolecular hydrogen-bonding interactions were introduced into pyrimidine–pyridine hybrid acceptors, then the TADF properties can be tuned by skillful manipulation of the positions of CH⋯N in the acceptor moiety. Both DPAc-4PyPM and DPAc-6PyPM exhibit a higher PLQY than DPAc-TPPM. Simultaneously, the kRISC realized for DPAc-4PyPM is nearly 5 or 10 fold higher than that of its analogues. The optimized sky-blue device using DPAc-4PyPM as a dopant demonstrated the maximum external quantum efficiency, current efficiency and power efficiency of 24.34%, 53.89 cd A−1 and 36.79 lm W−1, respectively, which is comparable to most of the previously reported pyrimidine-based TADF OLEDs.


 Please wait while we load your content...
Please wait while we load your content...