Surprising formation of quasi-stable Tc(vi) in high ionic strength alkaline media†
Abstract
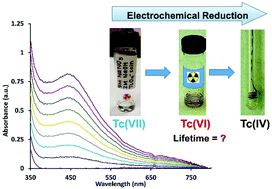
Despite decades of research, the reduction behavior of pertechnetate, TcO4−, is not well understood and represents one of the largest knowledge gaps in the transition metal series. Conventional wisdom presumes the reduction of TcO4− in aqueous systems to predominantly follow either a rapid two electron, or three electron process to form either a TcV or TcIV species. This study for the first time demonstrates the importance of the TcVI species through systematic characterization by multiple spectroscopic techniques. The reduction of TcO4− was examined in a matrix of 5 M NaNO3 at 0–2 M NaOH. Results of the cyclic voltammetric evaluation of reduction to TcVI, Nernstian analysis of the visible spectroelectrochemical signal, and structural spectroscopic EPR and XPS analysis of the reduction product generated by bulk electrolysis consistently support an electron transfer stoichiometry corresponding to a 1e− reduction. This TcVI species is markedly more stable than had been previously considered, with decomposition kinetics that correspond to a half-life of 1.91 ± 0.07 days, fully 6 orders of magnitude longer than previously reported for an aqueous solution. These results reveal the importance of a TcVI intermediate species in high ionic strength alkaline solutions and significantly contribute to the understanding of the mechanism of TcO4− reduction and formation of low-valent Tc species.


 Please wait while we load your content...
Please wait while we load your content...