An overview on Pd-based electrocatalysts for the hydrogen evolution reaction
Abstract
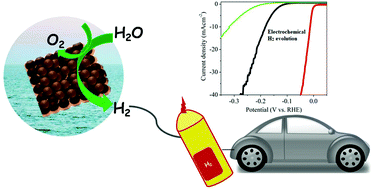
The most essential prerequisite for the hydrogen economy is sustainable hydrogen production. The electrochemical hydrogen evolution reaction is a well-studied electrochemical reaction which involves the reduction of protons for hydrogen production. It requires highly efficient and robust catalysts to lower the overpotential and energy consumption. Platinum (Pt) remains the first choice among electrocatalysts because of its excellent activity and high current density. Since Pt is limited by its high cost and scarcity, extensive research is devoted for the development of non-Pt-based cost-effective electrocatalysts. According to the volcano plot, palladium (Pd) can be used as a substitute for Pt towards the hydrogen evolution reaction (HER). Herein, we review various Pd-based electrocatalysts in the form of nanoparticles, alloys, bimetallics and intermetallics reported for the HER. In this review we have emphasized various synthesis techniques employed for these electrocatalysts and the strategies used for improving the catalytic performance. We conclude with a perspective on the future aspects of electrocatalysts based on Pd.
- This article is part of the themed collection: 2018 Inorganic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...