Crystal chemistry and thermoelectric transport of layered AM2X2 compounds†
Abstract
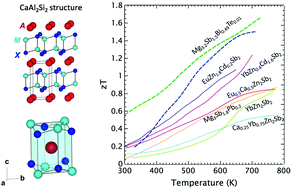
Compounds that crystallize in the layered CaAl2Si2 structural pattern have rapidly emerged as an exciting class of thermoelectric materials with attractive n- and p-type properties. More than 100 AM2X2 compounds that form this structure type – characterized by anionic M2X2 slabs sandwiched between layers of octahedrally coordinated A cations – provide numerous potential paths to chemically tune every aspect of thermoelectric transport. This review highlights the chemical diversity of this structure type, discusses the rules governing its formation and stability relative to competing AM2X2 structures (e.g., ThCr2Si2 and BaCu2S2), and attempts to bring some of the most recently discovered compounds into the spotlight. The discussion of thermoelectric transport properties in AM2X2 compounds focuses primarily on the intrinsic parameters that determine the potential for a high figure of merit: the band gap, effective mass, degeneracy, carrier relaxation time, and lattice thermal conductivity. We also discuss routes that have been used to successfully control the carrier concentration, including controlling the cation vacancy concentration, doping, and isoelectronic alloying (approaches that are highly interdependent). Finally, we discuss recent progress made towards n-type doping in this system, highlight opportunities for further improvements, as well as open questions that still remain.
- This article is part of the themed collection: 2018 Inorganic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...