Synthesis of a ROS-responsive analogue of poly(ε-caprolactone) by the living ring-opening polymerization of 1,4-oxathiepan-7-one†
Abstract
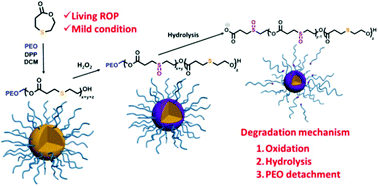
We report the synthesis of a poly(ε-caprolactone) (PCL) analogue containing thioether groups in the main chain by living ring-opening polymerization (ROP) of 1,4-oxathiepan-7-one (OTO). OTO was synthesized by a one-pot two-step method, and the ROP of OTO was conducted at 30 °C in dichloromethane with benzyl alcohol (BnOH) as the initiator and diphenyl phosphate (DPP) as the catalyst. The livingness of the polymerization was verified by polymerization kinetics and chain-extension experiment with ε-caprolactone (CL). Well-defined POTO and POTO-b-PCL with predetermined molecular weights and low dispersities were thus obtained. Furthermore, with monomethyl poly(ethylene oxide) (PEO) as the macroinitiator, diblock and triblock amphiphilic block copolymers, namely, PEO-b-POTO, PEO-b-POTO-b-PCL and PEO-b-PCL-b-POTO, were also prepared. Several small model molecules mimicking the β-thioether ester moieties in POTO were synthesized, and their H2O2-responsive properties in aqueous media were investigated in detail by NMR and MS. Upon exposure to excess H2O2, the thioether moieties rapidly and exclusively converted to sulfoxides within a few hours, and then hydrolysis of the ester groups adjacent to the sulfoxide occurred together with elimination reactions. Finally, using PEO114-b-POTO20 as an example, the H2O2-oxidized disassembly of the polymer micellar nanoparticles in aqueous solution was investigated by NMR, TEM, GPC, and DLS. We confirmed that although the oxidation of the thioether groups enhanced the hydrophilicity of POTO and contributed to the disassembly of the PEO114-b-POTO20 nanoparticles, the main driving force of nanoparticle disassembly was the oxidation-promoted hydrolysis of the POTO block.


 Please wait while we load your content...
Please wait while we load your content...