Solubility and size of polymer nanoparticles †
Abstract
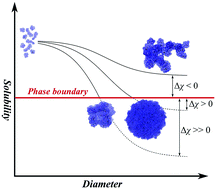
The solubility of polymer nanoparticles is a complex phenomenon dependent on solvent–solute and solute–solute interactions. Contrary to phase separation in standard polymerization reactions, which is a well established research area, the relationship between the solubility of polymer nanoparticles and the resulting diameter of the nanoparticles has been largely overlooked. Herein we demonstrate that the absolute size of polymer nanoparticles can be predicted (and controlled) by varying the relevant parameters of the polymerization conditions that influence the solubility and Flory parameter, χs, p. The position of the spinodal, associated with a given χs, p equivalent and determined with a simple thermodynamic model, allows an absolute value, Δχspinodal, to be applied in predicting polymer dimensions. The hydrodynamic diameter of particles at the primarily observed fraction was found to be dependent on D (nm) = −74Δχspinodal + 367 nm, where Δχspinodal must be positive for successful separation. Variation with total polymer fraction over a limited range can also be observed to follow a trend of approximately D (nm) = 173 ln[(xN)210−36/Δχspinodal] − 193 nm, thus giving a more general description of polymerization. We also assert the importance of separating spinodal-character phase separation from binodal-character phase separation in polymer nanoparticle synthesis. To the best of our knowledge this is the first successful Flory–Huggins based thermodynamic model of polymer nanoparticles, and should provide a useful guide to predictive design of future nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...