Nanocellulose/TiO2 composites: preparation, characterization and application in the photocatalytic degradation of a potential endocrine disruptor, mefenamic acid, in aqueous media†
Abstract
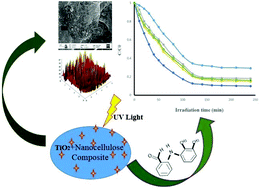
Nanocellulose (NC)-supported TiO2 nanoparticles, termed NCTs, were prepared by an ultrasonic impregnation method. The as-synthesized NCTs were thoroughly characterized and studied for the photodegradation of mefenamic acid (MEF), an anthranilic acid derivative drug. The adsorption potential of NCTs increased with TiO2 loading and 10 wt% TiO2 NCT showed the highest sorption potential. Adsorption kinetics of MEF onto NC and NCTs indicated that the equilibrium was reached within 50 min. A pseudo-second-order model clearly represented the experimental kinetic data and demonstrated that the MEF sorption was mainly chemisorption. Equilibrium sorption analysis indicated that the adsorption followed the Langmuir model with a monolayer sorption capacity of 22.43 mg g−1 for 10 wt% TiO2 NCT. The photocatalytic degradation rates of NCTs were identical with respect to their adsorption capacities, and the apparent rate constant (kapp) values indicated that the amount of TiO2 in NCTs played a vital role in the degradation of MEF. Furthermore, 10 wt% TiO2 NCT showed excellent catalytic activity and reusability even after five cycles of photodegradation.


 Please wait while we load your content...
Please wait while we load your content...