A facile tandem decyanation/cyanation reaction of α-iminonitriles toward cyano-substituted amides†
Abstract
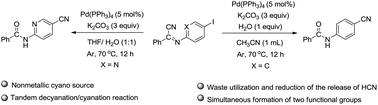
A new tandem decyanation/cyanation reaction of α-iminonitriles has been developed. A variety of cyano-substituted aryl amides and heteroaryl amides are synthesized in good yields. Both electron-rich and electron-deficient groups are compatible with the standard conditions. This reaction features a nonmetallic cyano source, tandem decyanation and cyanation reaction, waste utilization of the HCN from the hydrolysis of α-iminonitriles, formation of two important functional groups in one-step operation, etc.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...