Total synthesis of (±)-chondrosterin I using a desymmetric aldol reaction†
Abstract
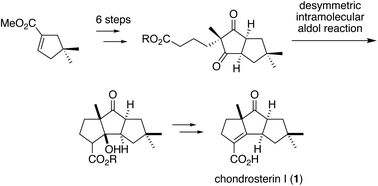
The first total synthesis of racemic chondrosterin I was accomplished. The synthetic features include a Michael addition to incorporate a nitro alkane moiety, an oxidative Nef reaction, intramolecular cyclization of the γ-ketoester derivative, and a desymmetric intramolecular aldol reaction of the meso diketoester compound. The present strategy will be applicable to the synthesis of an optically active form by asymmetric desymmetrization.


 Please wait while we load your content...
Please wait while we load your content...