Inverse-electron-demand Diels–Alder reactions of α,β-unsaturated hydrazones with 3-methoxycarbonyl α-pyrones†
Abstract
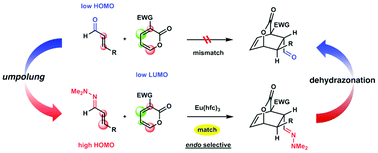
Inverse-electron-demand Diels–Alder reactions of 3-electron-withdrawing group substituted α-pyrones with α,β-unsaturated hydrazones as electron-rich counterparts are catalyzed by Eu(hfc)3 to afford bicyclic lactone cycloadducts. This is an example of umpolung cycloaddition based on functional transformation of carbonyls to hydrazones. A subsequent dehydrazonation reaction enables indirect synthesis of carbonyl group-containing bicyclic lactones, which cannot be easily obtained by the cycloaddition of α-pyrones and enals.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...