Gold(i)-catalysed high-yielding synthesis of indenes by direct Csp3–H bond activation†
Abstract
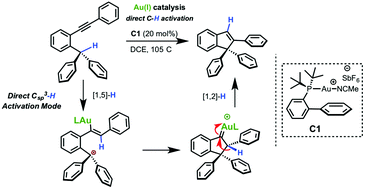
A catalytic, practical and high-yielding procedure for the synthesis of indenes by direct Csp3–H activation under gold(I) catalysis was developed. The scope of the protocol was determined by synthesizing some electron-neutral, electron-poor as well as electron-rich derivatives including the dibenzofurane and carbazole heterocycles. The mechanism of this reaction was elucidated by theoretical calculations using a ONIOM(M08-HX/mixed-basis:PM6) hybrid scheme. Thereby we found a pericyclic transformation involving a [1,5]-H shift generating a gold(I)–carbene that evolves to the indene derivative. In comparison with several reports, our protocol presents a direct activation of the Csp3–H bond.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...