Synthesis of 2-substituted 3-chlorobenzofurans via TMSCl-mediated nucleophilic annulation of isatin-derived propargylic alcohols†
Abstract
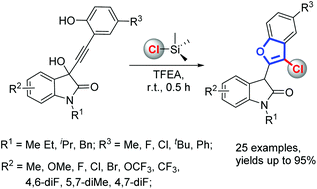
A TMSCl-mediated cascade annulation of isatin-derived propargylic alcohols for the synthesis of 2-substituted 3-chlorobenzofurans is now reported. Mechanistic investigations showed that this proceeded through a sequential Meyer–Schuster rearrangement/nucleophilic addition/intramolecular annulation. TMSCl not only acts as a promoter, but also acts as a chlorine source in this protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...