Synthesis of imidazopyridine-fused indoles via one-pot sequential Knoevenagel condensation and cross dehydrogenative coupling†
Abstract
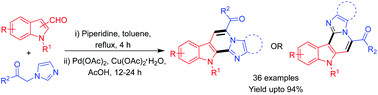
A simple and efficient strategy for the synthesis of imidazopyridine-fused indoles has been developed that involves one-pot sequential Knoevenagel condensation of readily available active methylene azoles with N-substituted-1H-indole-3-carboxaldehydes or N-substituted-1H-indole-2-carboxaldehydes followed by palladium-catalyzed intramolecular cross dehydrogenative coupling reaction. A series of 36 derivatives was prepared by using this strategy. The products were obtained in moderate to excellent (32–94%) yields and showed broad substrate scope with tolerance of various functional groups and was amiable for gram scale preparation without problems.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...