Base controlled diverse reactivity of allyl cyanide for synthesis of multi-substituted benzenes†
Abstract
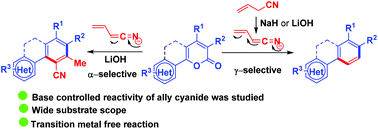
A base controlled regioselective 1,6-cyanoallylation of suitably functionalized 2H-pyran-2-ones has been demonstrated for the synthesis of various multi-substituted benzenes through a tandem process. We observed that lithium hydroxide provides a major product from α-attack and a minor product from γ-attack of allyl cyanide, while the use of sodium hydride as a base exclusively provides the product by γ-attack of allyl cyanide. We have also performed NMR experiments to understand the mechanistic pathway. The structure of the compound was confirmed by single crystal X-ray analysis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...