A catalytic asymmetric interrupted Nazarov-type cyclization of 2-indolylmethanols with cyclic enaminones†
Abstract
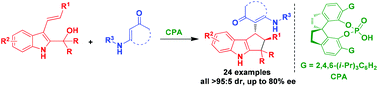
A chiral phosphoric acid-catalyzed asymmetric interrupted Nazarov-type cyclization of C3-alkenyl-substituted 2-indolylmethanols has been established by using cyclic enaminones as nucleophiles, which afforded chiral cyclopenta[b]indole derivatives with excellent diastereoselectivities and moderate to good enantioselectivities. This reaction will not only enrich the research contents of indolylmethanol-involved catalytic asymmetric transformations, but also advance the chemistry of catalytic asymmetric interrupted Nazarov-type cyclizations. In addition, this reaction will also provide a useful method for synthesizing chiral cyclopenta[b]indole derivatives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...