Total synthesis of the proposed structure of talarolide A†
Abstract
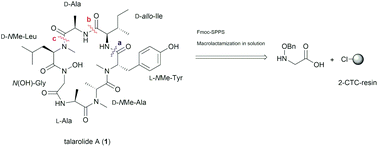
The proposed structure of talarolide A, a cycloheptapeptide featuring a hydroxamate moiety within the peptide backbone, was successfully synthesized. An initial attempt to synthesize a linear peptide precursor containing a C-terminal N-benzyloxy glycine residue was problematic due to an unreported on-resin reduction of N-benzyloxy glycine to glycine. After repositioning the peptide cyclization point, a new linear peptide sequence was successfully prepared using Fmoc-solid-phase peptide synthesis. Subsequent solution-phase cyclization and removal of protecting groups furnished the synthetic talarolide A in good yield. Despite the mismatch of the NMR data between the synthetic talarolide A and the natural product, a detailed structural analysis using 2D NMR spectroscopy, together with re-synthesis of the same synthetic material using two additional cyclization sites, confirmed that our synthetic product has the reported structure of talarolide A.
- This article is part of the themed collections: Synthetic methodology in OBC and Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...