Metal-free, base promoted sp2 C–H functionalization in the sulfonamidation of 1,4-naphthoquinones†
Abstract
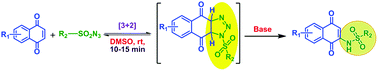
A novel, metal-free, base promoted sp2 C–H functionalization in the sulfonamidation of 1,4-naphthoquinones via a [3 + 2] cycloaddition reaction using sulfonyl azides under mild reaction conditions is reported. In this straightforward atom- and step-economical protocol, the active alkene moiety of quinone undergoes a thermal azide–alkene [3 + 2] cycloaddition followed by proton abstraction, ring opening and elimination of a nitrogen molecule to form sulfonamidation products in good yield and all the synthesized sulfonamidation derivatives exhibit good absorption and emission characteristics. In addition, the electrochemical properties of both 1,4-naphthoquinone and menadione sulfonamidation derivatives are studied and significant redox potentials are observed. Other important features of this methodology are readily accessible and easy to handle starting materials, milder conditions, reaction with a wide range of substrates and shorter reaction times with good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...