Total synthesis and structural revision of (±)-nidemone†
Abstract
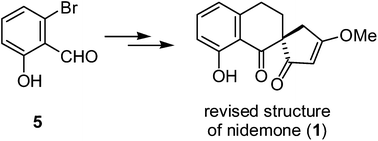
Total synthesis of the revised structure of (±)-nidemone has been accomplished from 6-bromo-2-hydroxybenzaldehyde (5) in either six or eight synthetic steps in 6% and 10% overall yield, respectively, without using any protecting group. The revised structure of nidemone was confirmed by single-crystal X-ray diffraction analysis. Sonogashira coupling, regioselective hydrogenation, and an intramolecular Stetter reaction were the key steps in the synthesis.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...