Synthetic access to isoxazoline-functionalized isoquinolines via microwave-assisted iminoxyl radical-participated cascade cyclization of vinyl isocyanides†
Abstract
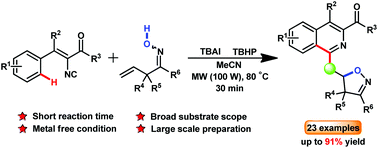
A convenient microwave-assisted method for the synthesis of isoxazoline-functionalized isoquinolines via the n-Bu4NI (TBAI) catalyzed radical cascade cyclization of vinyl isocyanides with β,γ-unsaturated ketoximes has been described. The methodology presented here achieves a wide substrate scope and good to excellent yields. A radical mechanism is proposed, which is supported by the intermolecular competing kinetic isotope effect (KIE) experiment and the radical trapping experiment.


 Please wait while we load your content...
Please wait while we load your content...