A macrocyclic receptor containing two viologen species connected by conjugated terphenyl groups†
Abstract
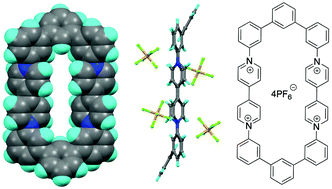
A macrocyclic receptor molecule containing two viologen species connected by conjugated terphenyl groups has been designed and synthesised. The single-crystal X-ray structure shows that the two viologen residues have a transannular N⋯N separation of ca. 7.4 Å. Thus, the internal cavity dimensions are suitable for the inclusion of π-electron-rich species. The macrocycle is redox active, and can accept electrons from suitable donor species including triethylamine, resulting in a dramatic colour change from pale yellow to dark green as a consequence of the formation of a paramagnetic bis(radical cationic) species. Cyclic voltammetry shows that the macrocycle can undergo two sequential and reversible reduction processes (E1/2 = −0.65 and −0.97 V vs. Fc/Fc+). DFT and TD-DFT studies accurately replicate the structure of the tetracationic macrocycle and the electronic absorption spectra of the three major redox states of the system. These calculations also showed that during electrochemical reduction, the unpaired electron density of the radical cations remained relatively localised within the heterocyclic rings. The ability of the macrocycle to form supramolecular complexes was confirmed by the formation of a pseudorotaxane with a guest molecule containing a π-electron-rich 1,5-dihydroxynaphthalene derivative. Threading and dethreading of the pseudorotaxane was fast on the NMR timescale, and the complex exhibited an association constant of 150 M−1 (±30 M−1) as calculated from 1H NMR titration studies.


 Please wait while we load your content...
Please wait while we load your content...