An organocatalyzed Stetter reaction as a bio-inspired tool for the synthesis of nucleic acid-based bioconjugates†
Abstract
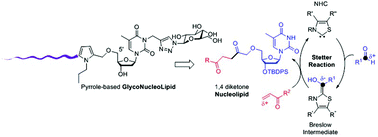
An N-Heterocyclic Carbene (NHC) catalyzed biomimetic Stetter reaction was applied for the first time as a bioconjugation reaction to sensitive nucleoside-type biomolecules to provide original pyrrole linked nucleolipids. A versatile approach allowed the functionalization of thymidine at the three reactive positions (O-5′, O-3′ and N-3) providing a structural diversity oriented synthesis. This strategy was applied to the synthesis of an original glyconucleolipid amphiphile in the hope that the pyrrole aromatic moiety would induce additional self-assembling properties.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...