Construction of bridged cyclic N,O-ketal spirooxindoles through a Michael addition/N,O-ketalization sequence†
Abstract
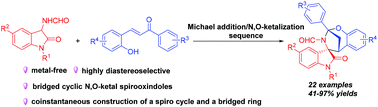
The first highly diastereoselective TfOH-catalyzed Michael addition/N,O-ketalization sequence of 3-aminooxindoles and ortho-hydroxychalcones was achieved, delivering a wide range of bridged cyclic N,O-ketal spirooxindoles with complex and strained structures in 41–97% yields. Moreover, a gram-scale experiment and some chemical conversions were conducted to further demonstrate the synthetic value.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...