An intramolecular oxa-Michael reaction on α,β-unsaturated α-amino-δ-hydroxycarboxylic acid esters. Synthesis of functionalized 1,3-dioxanes†
Abstract
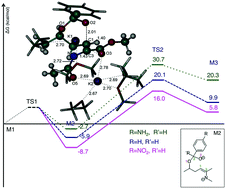
A highly diastereoselective intramolecular oxa-Michael reaction on α,β-unsaturated α-amino-δ-hydroxycarboxylic acid esters is presented; 1,3-dioxanes functionalized in positions 2,4 and 6 were obtained in good yields and with excellent selectivities; an experimental and computational study was carried out to understand the reaction course in terms of yields and selectivities. This reaction proceeds under mild reaction conditions using highly electrophilic aldehydes and ketones.


 Please wait while we load your content...
Please wait while we load your content...