An approach towards the synthesis of novel fused nitrogen tricyclic heterocyclic scaffolds via GBB reaction†
Abstract
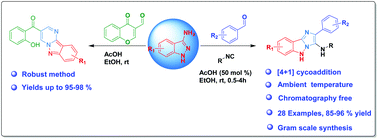
A concise and efficient one-pot synthesis of novel N-fused tricyclic derivatives has been developed by using the Groebke–Blackburn–Bienaymé (GBB) reaction, which involved the reaction of 3-amino-1H-indazoles, aldehydes and isonitriles to afford 2-aryl-5H-imidazo[1,2-b]indazol-3-amine derivatives via a formal [4 + 1] cycloaddition reaction. Furthermore, we describe an unprecedented reaction of chromone-3-carboxaldehydes with 3-amino-1H-indazoles to afford (2-hydroxyphenyl)(pyrimido[1,2-b]indazol-3-yl)methanones in one-pot at ambient temperature. This protocol features a robust method for the one-step construction of new tricyclic rings, column chromatography free methods with a clean reaction profile, high yields, operational simplicity and it tolerates a diverse collection of reactants.


 Please wait while we load your content...
Please wait while we load your content...