Asymmetric synthesis of (−)-sedacryptine through a diastereoselective Mannich reaction of N,O-acetals with ketones†
Abstract
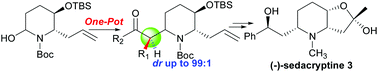
An efficient diastereoselective approach to access the 3-hydroxy-2,6-disubstituted piperidine scaffold 1 has been developed through the Mannich process involving N,O-acetal (2S,3R)-6 and ketones in excellent yield with high diastereoselectivity (dr > 99 : 1). In addition, the utility of this convenient one-pot process is demonstrated by the asymmetric synthesis of (−)-sedacryptine 3.


 Please wait while we load your content...
Please wait while we load your content...