Iodine mediated oxidative cross-coupling of unprotected anilines and heteroarylation of benzothiazoles with 2-methylquinoline†
Abstract
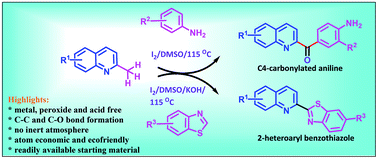
Iodine-promoted oxidative C–H/C–H cross-coupling of unprotected anilines and 2-methylquinoline to furnish C4-carbonylated aniline (4-aminophenyl)(quinoline-2-yl) methanones in moderate to good yields has been demonstrated. This work provides the first site-selective approach for the synthesis of free amino groups containing methanones including unprecedented C–H functionalization rather than the N–H functionalization of unprotected anilines via the Kornblum oxidation of 2-methylquinoline. Furthermore, we noticed that the incorporation of KOH under standard conditions provides 2-heteroarylbenzothiazoles from benzothiazoles and 2-methylquinoline in good to excellent yields. These transformations do not require any transition metals or peroxides and tolerate various functional groups such as methoxy, hydroxy, bromo, chloro and nitro groups. Moreover, a plausible mechanistic pathway is proposed.


 Please wait while we load your content...
Please wait while we load your content...