Nanostructure to thermal property relationship of resorcinol formaldehyde aerogels using the fractal technique†
Abstract
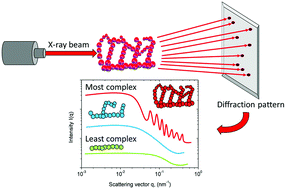
We characterized the morphological features of an organic resorcinol-formaldehyde (RF) aerogel and correlated each feature to the thermal insulation properties. Several RF aerogels were synthesized with different morphological features and structural assemblies. This was done by changing the catalyst percentages and the dilution ratios at the polymerization stage. Then, each morphological feature was assessed and categorized using two scales: the macro scale and the micro scale. We found that the macro-features were independent of the catalyst percentages and depended only on the dilution ratios. By contrast, the micro-features were highly sensitive to any changes during the polymerization process. These changes altered the samples’ three main micro-structural factors: (i) the structural assembly, (ii) the porous structure, and (iii) the fractal parameters. Thus, we characterized and quantified each component within these areas. Then, we assessed the structure's heat transfer modes and classified them as follows: (i) solid conductivity through the solid particles, (ii) gas conductivity through the gas molecules, and (iii) thermal radiation. We identified the morphological features in our RF samples and correlated them to each mode of the heat transfer. For example, the samples’ solid conductivity was highly dependent on the fractal parameters of our structure; that is, the particles’ roughness, the structural complexity, and the structural homogeneity. For those samples with extremely rough particles and a complex structure, the solid conductivity reached the lowest possible point. We also found that the total thermal conductivity was mainly controlled by the micro-morphological features, and that the solid conductivity was the most dominant heat transfer mode.


 Please wait while we load your content...
Please wait while we load your content...