New archetypes in self-assembled Phe-Phe motif induced nanostructures from nucleoside conjugated-diphenylalanines†
Abstract
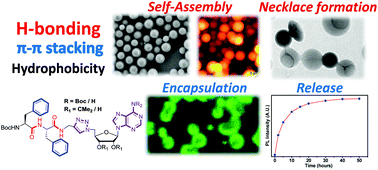
During the last two decades, the molecular self-assembly of the short peptide diphenylalanine (Phe-Phe) motif has attracted increasing focus due to its unique morphological structure and utility for potential applications in biomaterial chemistry, sensors and bioelectronics. Due to the ease of their synthetic modifications and a plethora of available experimental tools, the self-assembly of free and protected diphenylalanine scaffolds (H-Phe-Phe-OH, Boc-Phe-Phe-OH and Boc-Phe-Phe-OMe) has unfurled interesting tubular, vesicular or fibrillar morphologies. Developing on this theme, here we attempt to examine the effect of structure and properties (hydrophobic and H-bonding) modifying the functional C-terminus conjugated substituents on Boc-Phe-Phe on its self-assembly process. The consequent self-sorting due to H-bonding, van der Waals force and π–π interactions, generates monodisperse nano-vesicles from these peptides characterized via their SEM, HRTEM, AFM pictures and DLS experiments. The stability of these vesicles to different external stimuli such as pH and temperature, encapsulation of fluorescent probes inside the vesicles and their release by external trigger are reported. The results point to a new direction in the study and applications of the Phe-Phe motif to rationally engineer new functional nano-architectures.


 Please wait while we load your content...
Please wait while we load your content...