Novel nanocomposite membrane based on Fe3O4@TDI@TiO2–SO3H: hydration, mechanical and DMFC study
Abstract
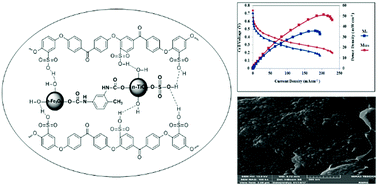
In this paper, a sulfonated poly(ether ether ketone)/SO3H-functionalized magnetic-titania (SPEEK/Fe3O4@TDI@TiO2–SO3H) nanocomposite membrane is synthesized with the aim of reducing methanol permeability as well as improving the proton conductivity and selectivity of pristine polymer to be used instead of Nafion in a direct methanol fuel cell (DMFC). The introduced nanocomposite membrane is prepared by solution casting of SPEEK in dimethyl acetamide (DMAc) solvent and dispersing various percentages of Fe3O4@TDI@TiO2–SO3H in the polymer matrix. The different properties of the membranes were investigated by ion exchange capacity, water uptake, mechanical stability, proton conductivity, methanol permeability, selectivity and a DMFC test. The catalytic properties of Fe3O4@TDI@TiO2–SO3H nanoparticles improve the power density of DMFC with increasing reaction kinetics in the membrane electrode assembly (MEA). According to the obtained results, a nanocomposite membrane (with optimum 5 wt% nanoparticles) has low methanol permeability (3.35 × 10−7 cm2 s−1), high proton conductivity (0.081 S cm−1), high mechanical stability (34.87 MPa) and high power density (51.27 mW cm−2), which makes it a suitable membrane for DMFCs.


 Please wait while we load your content...
Please wait while we load your content...