Bipolar behaviour of salt-bridges: a combined theoretical and crystallographic study†
Abstract
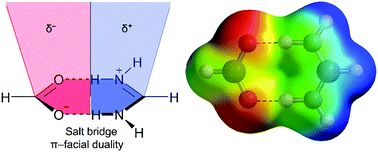
Crystal structures of aminopyridine–succinate salts (1–3) were solved by single crystal X-ray diffraction where salt bridge (SB) interactions between the succinate and protonated aminopyridine dominated their supramolecular architecture. X-ray crystallography reveals that compounds 1 (hydrogensuccinate 2-amino-5-methylpyridinium salt) and 2 (hydrogensuccinate 2-amino-4-methylpyridinium salt) exhibit C–H⋯SB⋯π+ assemblies, while compound 3 (succinato-aminopyridinium salt) displays unique N–H⋯SB⋯lone pair (l)p networks in the solid state. These unexplored extended networks are described and investigated for the first time in crystalline structures. Our study also describes the duality of the salt bridge in establishing π-facial interactions with electron rich and/or electron poor moieties depending on the positive or negative nature of the SB counterpart that interacts with the SB surface. The theoretical study combines an energetic investigation of the noncovalent forces that contribute to the extended supramolecular network and the characterization of the diverse interactions by means of Bader's theory of “atoms in molecules” (AIM) and the NCIplot index.


 Please wait while we load your content...
Please wait while we load your content...