Effect of electrode material and electrolysis process on the preparation of electrolyzed oxidizing water†
Abstract
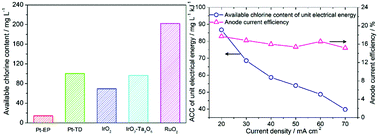
Electrolyzed oxidizing water (EO water) bactericide is an indirect electrochemical sterilization technology, which is characterized by broad-spectrum, rapid and powerful sterilization. EO water, with a certain amount of available chlorine content (ACC), is generated by electrolysis of an extremely dilute NaCl solution. It is very important to study the preparation process of EO water, including electrode material and electrolytic process. In this paper, the effect of electrode material (platinum, iridium or ruthenium) on the physical and chemical parameters of EO water was investigated first. The effect of electrode composition and roasting temperature on the ACC of EO water was rigorously analyzed. The sterilization effect of EO water produced by different electrode materials was further discussed. In addition, the accelerated service lifetime of the electrode and exchange electrode polarity electrolysis were also investigated. Next, for the electrolysis process, the effects of ion exchange membrane type, current density and electrolyte concentration on the ACC of EO water, anode current efficiency and energy consumption were also studied. Finally, the stability of EO water, that is, the influence of illumination, heating and stirring on the physical and chemical parameters of EO water, was also observed in detail.


 Please wait while we load your content...
Please wait while we load your content...