The dual reactivity of Weinreb amides applied to the late-stage divergent functionalisation of meso pyrrolidines†
Abstract
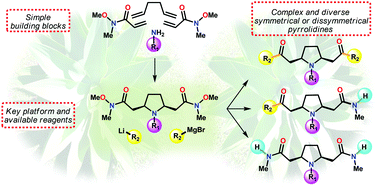
By exploiting the dual reactivity of a Weinreb amide moiety, i.e. nucleophilic addition or N–O reductive cleavage, a series of diversified symmetrical and dissymmetrical pyrrolidine analogues of lobelanine were synthesized from simple and readily available building blocks. Diversity is introduced at a late stage by using a wide variety of readily available organolithium and organomagnesium reagents, and chemoselective orientation can be achieved due to the steric encumbrance of both the reaction partners, the liganding ability of the starting meso pyrrolidine, and the nature of the organometallic species. This work constitutes the first example that takes advantage of the side reaction of Weinreb amide decomposition for the desymmetrization of a meso platform bearing two remote and highly flexible N-methoxy-N-methylamide arms through a probable beneficial metal-templated arrangement.


 Please wait while we load your content...
Please wait while we load your content...