Triple detection modes for Hg2+ sensing based on a NBD-fluorescent and colorimetric sensor and its potential in cell imaging†
Abstract
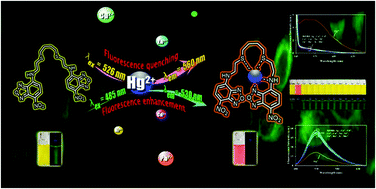
A new fluorescent sensor (DiNBD) was successfully synthesized; the sensor consisted of two 7-nitrobenzo-2-oxa-1,3-diazolyl (NBD) units that were covalently attached to 2-(4-(2-aminoethylthio)butylthio)ethanamine by a conventional two-step synthesis. It could serve as a colorimetric and fluorescent sensor for Hg2+ in aqueous acetonitrile solutions with high selectivity over other competitive metal ions. Upon Hg2+ titration, the sensor exhibited “ON–OFF” fluorescence quenching at 526 nm (λex 458 nm) and “OFF–ON” fluorescence enhancement at 560 nm (λex 530 nm), which were accompanied by a colorimetric change from yellow to red. The Hg2+ detection limit of the sensor was 0.7 ppb, which is lower than the permitted level of Hg2+ in drinking water regulated by US EPA and WHO. Additionally, the fluorescence quenching and fluorescence enhancement of the sensor in the presence of Hg2+ could provide a precise determination of Hg2+ concentration due to their self-calibration. Moreover, the DiNBD fluorescent sensor could be used to determine Hg2+ in real samples and living cells, which could widen the applications of the developed sensor.


 Please wait while we load your content...
Please wait while we load your content...