Chiral copper-salen complex grafted over functionalized mesoporous silica as an efficient catalyst for asymmetric Henry reactions and synthesis of the potent drug (R)-isoproterenol†
Abstract
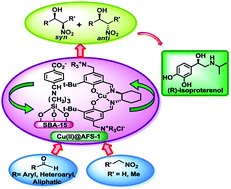
Synthesis of enantiomerically pure drug molecules using functionalized mesoporous materials bearing a chiral moiety is a longstanding goal in heterogeneous catalysis. Herein, we report an efficient and enantioselective one-pot Henry reaction over a highly ordered functionalized mesoporous silica-supported chiral copper-salen catalyst, Cu(II)@AFS-1, in DCM at RT. The catalyst was characterized by UV-Vis, FT-IR spectroscopy, PXRD, N2 adsorption/desorption, HR-TEM, EPR, and thermogravimetric and elemental analyses. The diastereoselective Henry reaction of nitroethane was carried out by using this catalyst to obtain good yields (up to 96%) and ee (up to 95%) of β-nitroalcohols with acceptable dr (3.2 : 1). Additionally, the enantiomerically pure drug (R)-(−)-isoproterenol can be synthesized through the asymmetric Henry reaction of 3,4-dimethoxybenzaldehyde over this chiral mesoporous Cu-catalyst as a key step.


 Please wait while we load your content...
Please wait while we load your content...