New 2-(aryl/heteroaryl)-6-(morpholin-4-yl/pyrrolidin-1-yl)-(4-trifluoromethyl)quinolines: synthesis via Buchwald–Hartwig amination, photophysics, and biomolecular binding properties†
Abstract
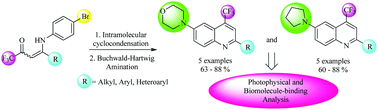
This work reports a successful synthesis of two new series of 2-(aryl/heteroaryl)-6-(morpholin-4-yl/pyrrolidin-1-yl)-(4-trifluoromethyl)quinolines via the Buchwald–Hartwig amination, at 60–88% yields, starting from 2-(aryl/heteroaryl)-6-bromo-4-trifluoromethyl-quinolines and heteroarylamines (morpholine and pyrrolidine). The 6-bromoquinoline precursors were obtained from an easy intramolecular cyclization reaction of (Z)-4-((4-bromophenyl)amino)-1,1,1-trifluoro-but-3-en-2-ones, which were previously prepared through the reaction of 4-methoxy-(aryl/heteroaryl)-1,1,1-trifluoroalk-3-en-2-ones with 4-bromoaniline. Photophysical analyses indicated intraligand and charge-transfer type transitions, in agreement with the aromatic structures of the heterocycle moieties. In the case of ct-DNA titrations (via the absorption and emission method), morpholinyl- and pyrrolydinyl-substituted quinolines had strong interactions with ct-DNA, which could be attributed to π-stacking and/or hydrogen-bonding interactions.


 Please wait while we load your content...
Please wait while we load your content...