Development and validation of a stability-indicating RP-HPLC method for simultaneous determination of dapagliflozin and saxagliptin in fixed-dose combination
Abstract
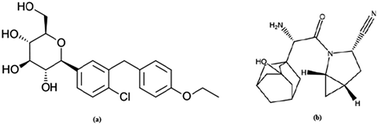
A simple and precise stability indicating method for the simultaneous estimation of dapagliflozin and saxagliptin in combined tablet dosage form was developed and validated using RP-HPLC. The chromatographic separation of the drugs was achieved with an Xterra C-18 analytical column (150 mm × 4.6 mm i.d., particle size 3.5 μ) using buffer and acetonitrile (53 : 47 v/v) as the mobile phase. The buffer used in mobile phase contained 20 mM sodium dihydrogen phosphate and its pH was adjusted to 5.5 ± 0.02 with orthophosphoric acid. The instrument was set at a flow rate of 1.2 mL min−1 at ambient temperature and the wavelength of the UV-visible detector was 230 nm. The method showed excellent linearity over a range of 2–14 μg mL−1 for all the drugs. The correlation coefficients for dapagliflozin and saxagliptin were noted to be 0.997 and 0.996, respectively. The mean recovery values were found to be 99.16% and 100.58%. The proposed method could be suitable for quantitative determination of these drugs in pharmaceutical preparations and also for quality control in bulk manufacturing. Stress testing, which covered acid, base, peroxide, photolytic and thermal degradation, was performed on each test to prove the specificity of the method and that the degradation was achieved. No interferences were observed from the stress degradation products. The F-test and t-test at 95% confidence level were applied to the data for statistical analysis.


 Please wait while we load your content...
Please wait while we load your content...