A joint experimental and theoretical study on the electro-optical properties of 1,6- and 1,7-fluorenyl disubstituted perylene diimide isomers†
Abstract
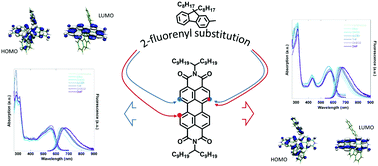
Core substituted perylene diimides (PDIs), obtained by introducing various functional groups into the perylenic bay positions, are well known as versatile materials for optoelectronic applications. The substitution of the perylene core affords mono-, di- and tri-substituted derivatives, governed by the regioisomeric mixture of the 1,6- and 1,7-disubstituted PDIs. Most often, the disubstituted isomers are used as a mixture because of the difficulty in separating them by conventional methods or time-consuming crystallization processes. In this work, from the regioisomeric mixture of bis[(9,9′-dioctylfluorenyl)]-N,N′-bis-(10-nonadecyl)-perylene-3,4,9,10-bis(dicarboximide), the 1,6-(PDI-F1) and 1,7-(PDI-F2) isomers have been easily isolated and separated by column chromatography and were subsequently characterized. The spectral features, electronic density distribution, and ground and excited state dipole moments of PDI-F1 have been examined and compared with those of PDI-F2. Several differences have been observed in the properties of the two PDI isomers. The PDI-F1 absorption spectrum shows a unique broad band spanning from 450 nm to 640 nm, while PDI-F2 exhibits the lowest energy absorption at 570 nm and a strong band at 420 nm. The absorption and emission properties in different solvents have evidenced that although both regioisomers show dipole moment values of the excited states higher than those of the corresponding ground states, the dipole moment variation upon excitation is different for each isomer. Cyclic voltammetry measurements revealed that PDI-F2 has a stronger electron accepting ability, as evidenced by lower reduction potentials. Complementary density functional theory calculations are also reported in order to rationalize their electronic and optical properties.


 Please wait while we load your content...
Please wait while we load your content...