Microcapsules from diverse polyfunctional materials: synergistic interactions for a sharp response to pH changes†
Abstract
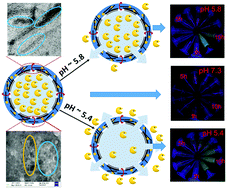
In the present article, we report the preparation of microcapsules from functionalized polymers, carbon nanotubes and silver nanoparticles, which are stabilized and interconnected by a cross-linking agent. These can be conveniently used to fine tune the release of important encapsulated materials like Vitamin B2 and anticancer drugs with very little variation of pH (e.g. pH 5.4 vs. pH 5.8) in the physiological range. It was observed that even though microcapsules can be formed with acid functionalized single walled carbon nanotubes (f-SWCNTs) or polymer–silver nanoparticle combinations, better stability and responsiveness were found with a polymer–silver nanoparticle–f-SWCNT combination. Optical microscopic studies (video and still pictures) showed that the microcapsules did not break even with an appreciable amount of disturbance by mechanical means (nitrogen gas flow) or the dropwise addition of acidified buffer/water, clearly indicating that the release phenomenon was happening through the gap produced by the nanomaterials rather than the breaking of the microcapsules. HRTEM and FESEM pictures showed those gaps and the composite natures of the different nanomaterials – clearly indicating that a possible synergistic effect is responsible for the superior stability and interlinking. ICP-OES analysis reveals the pH dependent release of the drugs and in vitro studies indicated that the prepared pH responsive microcapsules could be useful for pH-triggered targeted drug delivery applications.


 Please wait while we load your content...
Please wait while we load your content...