Following Ramachandran 2: exit vector plot (EVP) analysis of disubstituted saturated rings
Abstract
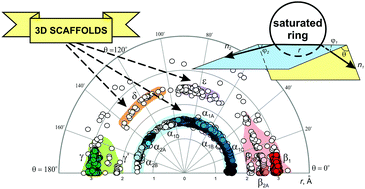
Analysis of disubstituted heteroaliphatic, as well as all common saturated rings using the exit vector plots (EVPs) tool is described, which is based on the Cambridge Structural Database (CSD) data. It is shown that the combined EVPs for saturated rings are similar to those observed for cycloalkanes (i.e. α, β, γ, and δ regions are found). In addition to that, a new region (ε) is found which is not accessible by simple cycloalkane derivatives. It is shown that while introducing saturated rings into the molecules is widely considered as an approach to increase their non-planarity, not many of them are truly three-dimensional; some recommendations are given to address this issue.


 Please wait while we load your content...
Please wait while we load your content...