Synthesis, characterization and cytotoxic activity evaluation of ginsengdiol oxidation and nitrogen hybrid derivatives†
Abstract
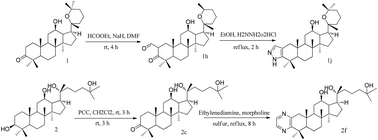
Panaxadiol (PD), a diol-type ginseng saponin, with a dammarane skeleton plays a potential role in the apoptosis of tumor cells. In this study, 28 oxidation and nitrogen hybrid derivatives of PD were synthesized, of which 20 were novel compounds. All the obtained compounds were screened for their cytotoxic activity in six cell lines. As compared with the positive control, some compounds showed better anti-proliferative activities while having much weaker effect on the growth of normal cells. Among them, ring-A fused pyrazoline of PD (1j) displayed impressive cytotoxic activity with IC50 9.62 ± 1.34, 11.65 ± 1.71, and 13.45 ± 1.60 μM against A549, HeLa and 8901 cell lines, respectively. Additionally, compound 2f exhibited the most potent activity with an IC50 value of 8.93 ± 1.11 μM against cell line A549. Therefore, our results indicated that 1j and 2f can be promising lead candidates for further studies.


 Please wait while we load your content...
Please wait while we load your content...