Synthesis and evaluation of 6-heteroarylamino-2,4,5-trimethylpyridin-3-ols as inhibitors of TNF-α-induced cell adhesion and inflammatory bowel disease†
Abstract
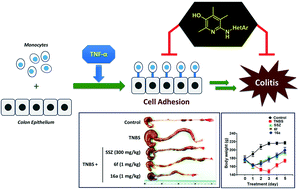
Inflammatory bowel disease (IBD) is an inflammatory disease of the gastrointestinal tract with complex pathogenesis. Here, we synthesized 6-heteroarylamino analogues to inhibit TNF-α-induced adhesion of monocytes to colon epithelial cells which are implicated in the initial inflammation process of IBD. The best analogue, 16a, showed IC50 = 0.29 μM, which is about five orders of magnitude better than that of 5-aminosalicylic acid (5-ASA), a positive control. Oral administration of 6f and 16a dramatically ameliorated 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colon inflammation in rat. The ameliorating effects were accompanied by a high level of recovery in colon and body weights and in the myeloperoxidase (MPO) level. Consistently, the compounds suppressed the expression of intercellular adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein 1 (MCP-1). Moreover, they significantly suppressed the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 while increasing the level of IL-10, an anti-inflammatory cytokine.


 Please wait while we load your content...
Please wait while we load your content...