Identification of karanjin isolated from the Indian beech tree as a potent CYP1 enzyme inhibitor with cellular efficacy via screening of a natural product repository†‡
Abstract
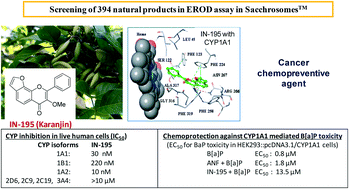
CYP1A1 is thought to mediate carcinogenesis in oral, lung and epithelial cancers. In order to identify a CYP1A1 inhibitor from an edible plant, 394 natural products in the IIIM's natural product repository were screened, at 10 μM concentration, using CYP1A1-Sacchrosomes™ (i.e. microsomal enzyme isolated from recombinant baker's yeast). Twenty-seven natural products were identified that inhibited 40–97% of CYP1A1's 7-ethoxyresorufin-O-deethylase activity. The IC50 values of the ‘hits’, belonging to different chemical scaffolds, were determined. Their selectivity was studied against a panel of 8 CYP-Sacchrosomes™. In order to assess cellular efficacy, the ‘hits’ were screened for their capability to inhibit CYP enzymes expressed within live recombinant human embryonic kidney (HEK293) cells from plasmids encoding specific CYP genes (1A2, 1B1, 2C9, 2C19, 2D6, 3A4). Isopimpinellin (IN-475; IC50, 20 nM) and karanjin (IN-195; IC50, 30 nM) showed the most potent inhibition of CYP1A1 in human cells. Isopimpinellin is found in celery, parsnip, fruits and in the rind and pulp of limes whereas different parts of the Indian beech tree, which contain karanjin, have been used in traditional medicine. Both isopimpinellin and karanjin negate the cellular toxicity of CYP1A1-mediated benzo[a]pyrene. Molecular docking and molecular dynamic simulations with CYP isoforms rationalize the observed trends in the potency and selectivity of isopimpinellin and karanjin.

 Please wait while we load your content...
Please wait while we load your content...