Design, synthesis and evaluation of indole derivatives as multifunctional agents against Alzheimer's disease
Abstract
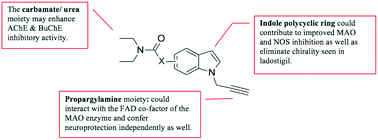
A series of indole derivatives was designed and synthesised to improve on activity and circumvent pharmacokinetic limitations experienced with the structurally related compound, ladostigil. The compounds consisted of a propargylamine moiety (a known MAO inhibitor and neuroprotector) at the N1 position and a ChE inhibiting diethyl-carbamate/urea moiety at the 5 or 6 position of the indole ring. In order to prevent or slow down the in vivo hydrolysis and deactivation associated with the carbamate function of ladostigil, a urea moeity was incorporated into selected compounds to obtain more metabolically stable structures. The majority of the synthesised compounds showed improved MAO-A inhibitory activity compared to ladostigil. The compounds possessing the propargylamine moiety showed good MAO-B inhibitory activity with 6 and 8 portraying IC50 values between 14–20 fold better than ladostigil. The ChE assay results indicated that the compounds have non-selective inhibitory activities on eeAChE and eqBuChE regardless of the type or position of substitution (IC50: 2–5 μM). MAO-A and MAO-B docking results showed that the propargylamine moiety was positioned in close proximity to the FAD cofactor suggesting that the good inhibitory activity may be attributed to the propargylamine moiety and irreversible inhibition as confirmed in the reversibility studies. Docking results also indicated that the compounds have interactions with important amino acids in the AChE and BuChE catalytic sites. Compound 6 was the most potent multifunctional agent showing better inhibitory activity than ladostigil in vitro on all enzymes tested (hMAO-A IC50 = 4.31 μM, hMAO-B IC50 = 2.62 μM, eeAChE IC50 = 3.70 μM, eqBuChE IC50 = 2.82 μM). Chemical stability tests confirmed the diethyl-urea containing compound 6 to be more stable than its diethyl-carbamate containing counterpart compound 8. Compound 6 also exerted significant neuroprotection (52.62% at 1 μM) against MPP+ insult to SH-SY5Y neural cells and has good in silico predicted ADMET properties. The favourable neuronal enzyme inhibitory activity, likely improved pharmacokinetic properties in vivo and the potent neuroprotective ability of compound 6 make it a promising compound for further development.

 Please wait while we load your content...
Please wait while we load your content...