C–H functionalization enabled stereoselective Ugi-azide reaction to α-tetrazolyl alicyclic amines†
Abstract
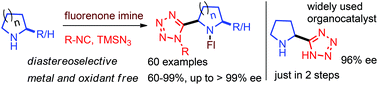
An unprecedented stereoselective C(sp3)–H functionalization enabled Ugi-azide (CH-Ugi-azide) reaction is reported. This novel reaction produces α-tetrazolyl N-heterocycles directly from N-heterocycles without involving pre-functionalization/pre-oxidation steps. Importantly, the stereoselective reaction involving chiral amines or chiral isocyanides allowed the expeditious syntheses of nucleoside analogs and α-tetrazolyl pyrrolidine in enantioenriched form.


 Please wait while we load your content...
Please wait while we load your content...