Organic ligand-free carbonylation reactions with unsupported bulk Pd as catalyst†
Abstract
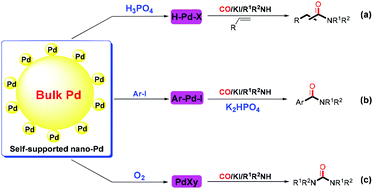
Herein, surprising results for bulk Pd-catalyzed carbonylation reactions are presented. Three types of carbonylation reactions can be realized efficiently under organic ligand-free conditions, namely, hydroaminocarbonylation of olefins, aminocarbonylation of aryl iodides and oxidative carbonylation of amines, which almost cover all the known mechanisms in carbonylation reactions. Notably, the bulk Pd catalyst system exhibited better catalytic activity than the classical homogeneous PdCl2/(2-OMePh)3P catalyst system. This study will create a momentous and new field of green carbonylation reactions.


 Please wait while we load your content...
Please wait while we load your content...