The role of the hydrogen evolution reaction in the solid–electrolyte interphase formation mechanism for “Water-in-Salt” electrolytes†
Abstract
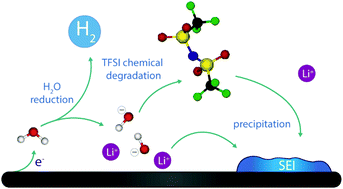
Aqueous Li-ion batteries have long been envisioned as safe and green energy storage technology, but have never been commercially realized owing to the limited electrochemical stability window of water, which drastically hampers their energy density. Recently, Water-in-Salt electrolytes (WiSEs) in which a large amount of organic salt is dissolved into water were proposed to allow for assembling 3 V Li-ion batteries. Hereby, our attention focused on the fate of water at the electrochemical interface under negative polarization and the potential reactivity of TFSI anions with products originating from the water reduction. Hence, combining analysis of bulk electrolytes with electrochemical measurements on model electrodes and operando characterization, we were able to demonstrate that hydroxides generated during the hydrogen evolution reaction can chemically react with TFSI and catalyze the formation of a fluorinated solid–electrolyte interphase (SEI) that prevents further water reduction. Mastering this new SEI formation path with the chemical degradation of TFSI anions mediated by the electrochemical reduction of water can therefore open new avenues for the future development of not only WiSEs but also Li batteries functioning in organic electrolytes.


 Please wait while we load your content...
Please wait while we load your content...