Beneficial use of rotatable-spacer side-chains in alkaline anion exchange membranes for fuel cells†
Abstract
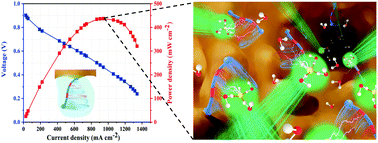
Side-chain-type polymer architectures have been extensively studied for development of highly conductive fuel cell membranes. However, the commonly used rigid, hydrophobic spacers (between the ionic end-group and polymer backbone) limit self-assembly of ionic side-chains and, therefore, ion transport. Herein, we report a flexible, hydrophilic side-chain-type anion exchange membrane (AEM), where ethylene oxide spacers are incorporated into imidazolium-containing cationic side-chains. AFM and SAXS analysis confirm that the flexible spacers facilitate self-assembly of the ionic side-chains to form continuous conducting channels. Most importantly, both in situ FTIR spectroscopy and molecular dynamic theory simulations indicate that the ethylene oxide spacers are capable of hydrogen bonding to both H2O molecules and hydrated OH− ions. This unique auxiliary function facilitates both ion and H2O transport during fuel cell operation. The resultant AEM exhibits a peak power density of 437 mW cm−2 at 65 °C when tested in a H2/O2 single-cell anion-exchange membrane fuel cell, which is among the highest reported for comparable side-chain-type AEMs.


 Please wait while we load your content...
Please wait while we load your content...