Group 10 metal–thiocatecholate capped magnesium phthalocyanines – coupling chromophore and electron donor/acceptor entities and its impact on sulfur induced red-shifts†‡
Abstract
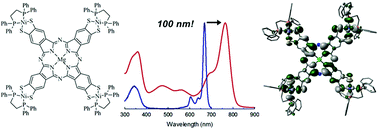
A new and facile method of generating thiolate groups at the phthalocyanine (Pc) β-position is presented as well the unique properties that these groups confer on the Pc ligand upon coordination of group 10 metals Ni, Pd and Pt(dppe) or SnMe3. In particular, the Q-band is shifted to almost 800 nm for all group 10 metals used, and the complexes show panchromatic absorption owing to new absorbance bands that appear between 400 and 650 nm. Enhanced intersystem-crossing for all transition metal coordinated Mg(Pc) complexes was demonstrated by the moderate to very high singlet oxygen quantum yields of 0.36, 0.76 and 0.91 for the Ni, Pd and Pt coordinating complexes, respectively, which show that the heavy metals have direct influence on the Pc π-system and inter-system-crossing (ISC). This was further confirmed by MO calculations, which show mixing of metal and ligand orbitals, as well as suggest that the Q-band transition has both π → π* and ligand-to-metal charge transfer characteristics. Furthermore, the origin of the Q-band red-shift was shown to be due to greater destabilization of the HOMO compared to LUMO/LUMO+1, thus decreasing the HOMO–LUMO band gap.


 Please wait while we load your content...
Please wait while we load your content...